The NIHR supports medtech research through commissioning and funding research projects and programmes. Through the innovation pathway, NIHR supports the development of medical devices and diagnostics. Companies can access these resources at any stage in their clinical development process.
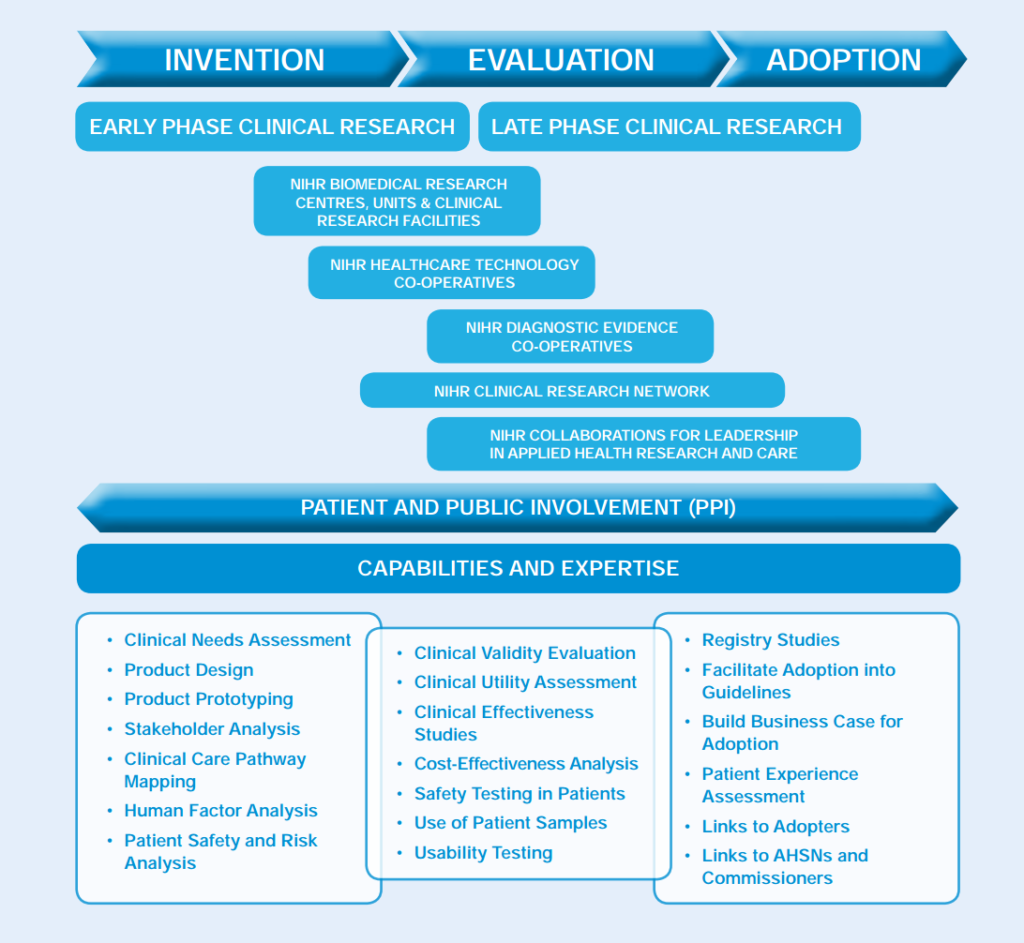
The NIHR infrastructure
BRCs and BRUs
NIHR Biomedical Research Centres (BRCs) and Units (BRUs) are based in the country’s leading hospitals and university partnerships. They conduct translational research to transform scientific breakthroughs into life-saving treatments for patients. NIHR BRCs and BRUs:
- host the country’s leading expert investigators and clinicians
- carry out research across a broad range of disease and therapeutic areas relevant to medtech development
- have experience in working with the medical device and diagnostic industry, with over 400 studies undertaken in 2014/15
CRFs
The NIHR supports 19 Clinical Research Facilities (CRFs) across the country, providing specialist clinical research staff who carry out commercial and non-commercial experimental medicine studies. NIHR CRFs:
- offer dedicated and purpose-built facilities
- support high-intensity studies and overnight stays
- provide medtech companies with assistance for their studies throughout the research process, from study design to data collection and management
HTCs
NIHR runs eight Healthcare Technologies Co-operatives (HTCs) across the country. The HTCs address clinical areas of themes of high morbidity and unmet need for NHS patients and healthcare technology users which have not benefited from a high degree of innovation to date. The HTCs are:
- centres of expertise to develop new concepts, demonstrate proof or principle and devise research protocols for new medical devices, healthcare technologies or technology-dependent interventions
- able to act as a catalyst for NHS ‘pull’ for the development of new technologies
- experienced in working collaboratively with patient groups, charities, industry and academics
- experienced in a wide range of clinical areas including brain injury, gastrointestinal and colorectal, wound treatment and trauma management
DECs
The Diagnostic Evidence Co-operatives (DECs) are focused on in vitro diagnostic devices. These are centres of expertise that support the generation of evidence on commercially available in vitro diagnostic devices (IVDs). This is necessary for the NHS and IVD manufacturers to enable patients to access the most appropriate treatments more quickly and to help the NHS make the best use of its resources. NIHR DECs are:
- able to form multidisciplinary collaborative teams composed of healthcare professionals, the NHS, the IVD industry, academic researchers, including health economists and patients
- developing world-class methodologies that can be used to demonstrate clinical utility, cost-effectiveness and other benefits of industry developed IVDs.
CLAHRCs
The NIHR Collaborations for Leadership in Applied Health Research and Care (CLAHRCs) focus on applied health research and are working with the NHS on implementation of new interventions and treatments. They are:
- developing and conducting applied health research relevant across the NHS, and transforming research findings into improved outcomes for patients
- providing evidence of NHS and patient acceptability of medical devices
HSRIC
The NIHR Horizon Scanning Research and Intelligence Centre (HSRIC) provides advance notice to health service policy makers and research funders of selected new and emerging health technologies that may require additional research or evaluation, consideration of clinical and cost impact, or modification of clinical guidance. The nIHR HSRIC:
- produces technology alerts of new and emerging devices, in vitro diagnostics, imaging techniques and procedures
- provides intelligence that may be of use to the research community and healthcare organisations including commissioners, hospital and community providers, health professionals and procurement staff, as well as patients
- works with manufacturers and developers to identify and prioritise emerging and new health technologies
Contact us for more information on NIHR and funding opportunities.


